The Lurking Threat of Henipaviruses
In this post, I am going to discuss Henipaviruses of the family Paramyxoviridae (which includes measles, mumps, and respiratory syncytial virus) because they are little-known but very lethal RNA viruses. They emerged in the late 20th and early 21st centuries and have the potential to cause large outbreaks in the future because the human population lacks immunity.
Introduction to Henipaviruses
The genus Henipavirus contains two types of viruses -- Hendra and Nipah viruses -- which give the genus its name. Hendra virus has only been found in Australia, while Nipah virus infections have occurred in Malaysia, Singapore, Bangladesh, India, and the Philippines. Nipah antibodies or RNA have been isolated from bats in these regions, as well as in Cambodia, Thailand, and Indonesia, suggesting that the virus is endemic in Southeast Asia 1. A third Henipavirus, Cedar virus, was identified in Australian bats in 2012. Although it can replicate in animal cell lines, it does not cause disease in ferrets or guinea pigs 2. The lack of pathogenicity may be because Cedar virus has fewer proteins that interfere with the host immune response than Nipah and Hendra viruses 3,4.
Nipah and Hendra viruses have only caused local outbreaks in Southeast Asia and Australia because the diseases progress quickly and do not cause many asymptomatic infections. Symptoms are generally severe, so infected people and animals can be easily identified and isolated. Human-to-human transmission was also not evident in many outbreaks, so Henipaviruses have low pandemic potential because they are not easily transmitted between people. However, viruses are evolving all the time, and Henipaviruses may develop the ability to transmit more efficiently among humans. Combined with their extreme lethality (>50% in most outbreaks), Henipaviruses may become more serious public health threats in the future.
Bats of the Pteropus genus harbor Nipah, Hendra, and Cedar viruses but do not show symptoms of disease when infected. As discussed in a previous post on zoonotic spillovers, bats have many immunological adaptations that allow them to tolerate lethal viruses without symptoms or death. Pteropus bats are found throughout Southeast Asia, Oceania, and Australia, and distributions of individual species overlap considerably.
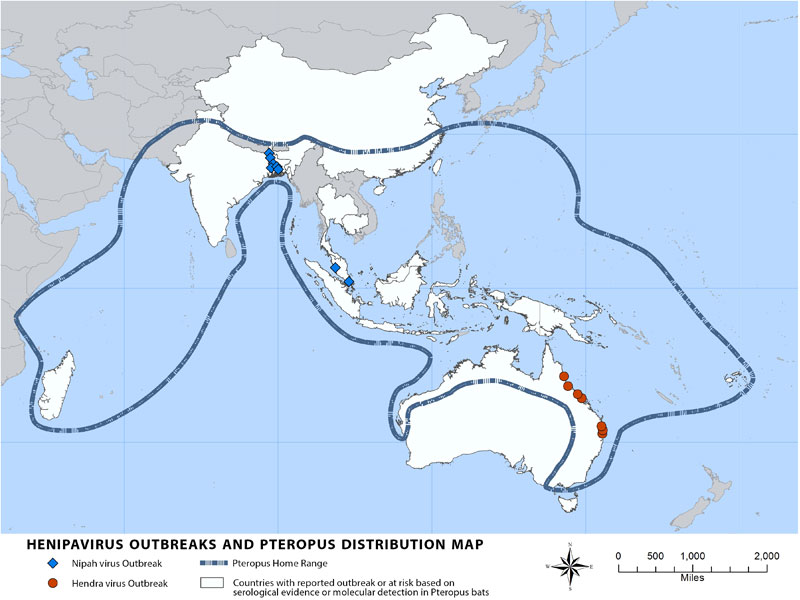
Source: Centers for Disease Control and Prevention
Hendra Virus Emergence
Hendra virus was first isolated in the suburb of Hendra in Brisbane, Queensland in August 1994. At a farm that raises racing horses, 20 horses were infected, as well as a trainer named Vic Rail, who later died. Infected horses all died or were euthanized to prevent future transmissions. The first horses were presumed to have a morbillivirus infection, similar to measles, but the virus was later reclassified from equine morbillivirus to Hendra virus in its own genus. It is believed that horses acquired the virus from contact with inanimate objects (fomites) contaminated with bat saliva or urine. Pteropus bats (also known as flying foxes) roost in large groups in trees, and if a horse simply sat underneath a roosting tree, it could have touched bat excrement or fruit dropped by the bats and contracted the virus.
Horse-to-horse transmission was evident in stables, but there have been no cases demonstrating human-to-human transmission of Hendra virus. The seven known humans who suffered from Hendra virus all had close contact with infected horses, typically during necropsies or while caring for sick horses. Australian handlers of captive and rehabilitating bats tested for antibodies to Hendra virus, which are indicative of a past infection, all returned negative. This suggests that bat-to-human transmission of Hendra virus does not yet occur. In Australia, at least 3 asymptomatic Hendra cases in dogs who had contact with horses were identified. Two were euthanized as a precaution, but it was unclear whether the dogs could have transmitted the virus to humans.
There have been reports of Hendra and Nipah virus infections apparently resolving, but then reactivating months or even years later. After a period of latency in the host, the virus may cause a secondary infection, which typically involves the brain and is fatal. One well-known case is that of Mark Preston, a farmer in Mackay near Brisbane. Mark helped his wife, Margaret, a veterinarian, perform necropsies of 2 horses in August 1994, and he was later hospitalized for meningitis. He apparently recovered, but the cause was unknown because Hendra virus was not identified until later that same month in Hendra. However, in October 1995, Mark developed encephalitis (brain inflammation) and died. He was retrospectively found to have had Hendra virus in his brain tissue, and the 2 horses from his farm tested positive for Hendra virus posthumously. He likely contracted the virus during the horse necropsies in 1994, but survived the first time (interestingly, Margaret never became sick) 5. The virus was not completely cleared from his body and remained until a stimulus caused it to increase either its replication or virulence.
Conditions Facilitating Hendra Virus Spillover
It is natural to wonder why Hendra virus (and other zoonotic spillovers) occurred when they did in time and space. This is due to a combination of favorable ecological factors. There are four species of flying fox native to Australia: Pteropus alecto, Pteropus scapulatus, Pteropus poliocephalus, and Pteropus conspicillatus. A 2011 study sampled roost sites, not individual bats, in northern Australia 6. They collected pooled urine samples from bat roosts and screened them for RNA encoding the matrix protein, which is likely to be similar across many Hendra virus isolates. The known ranges of the 4 bat species are shown in the figure below from the paper, along with the sampled roost sites and Hendra virus outbreaks:
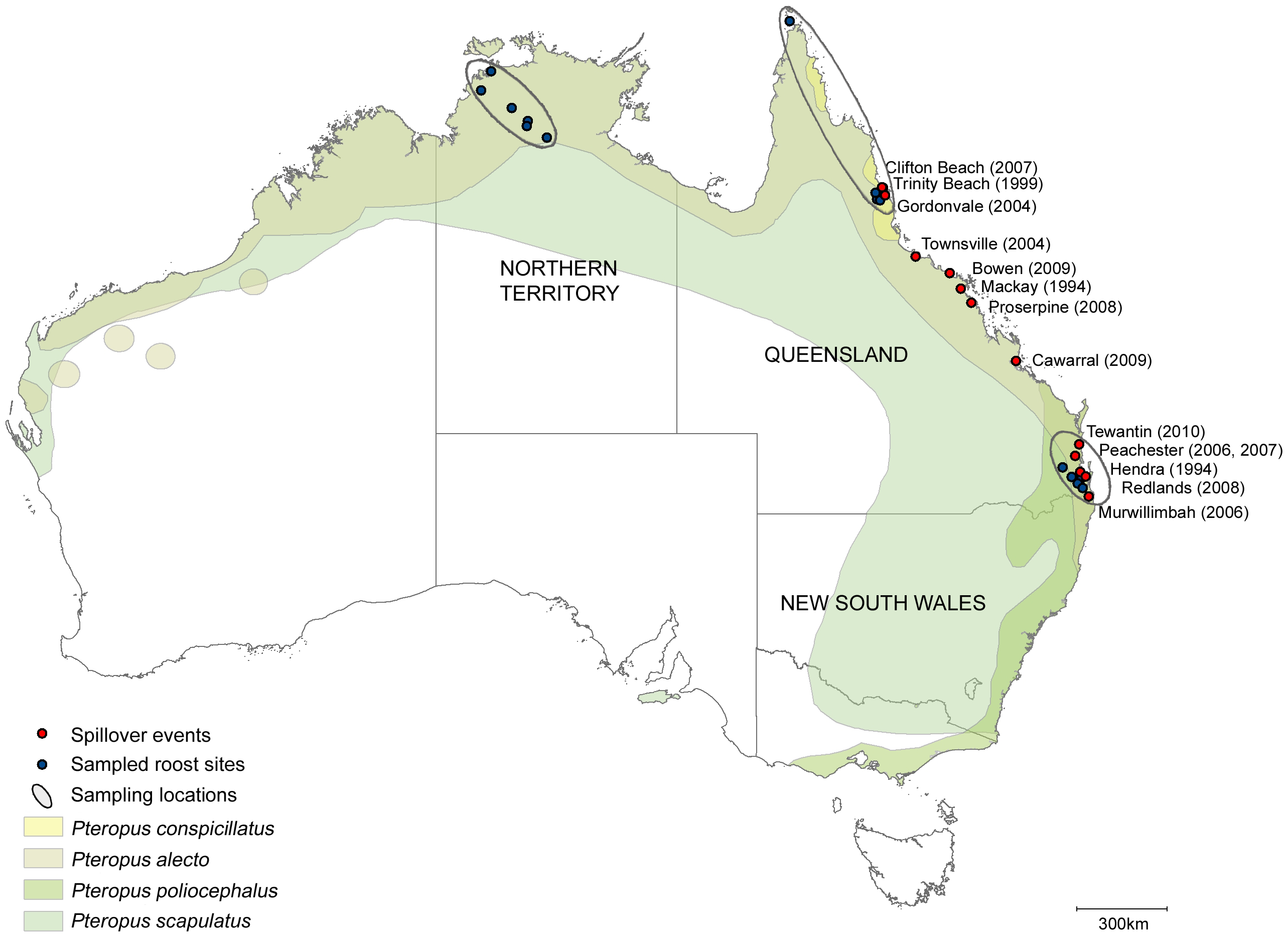
Figure 1, Field et al., 2011
Due to natural habitat clearing by humans in Australia, flying foxes are forced to look for food in areas densely populated by humans and our animals 7. This makes spillovers all the more likely as bats begin to feed on fruits and nectar in gardens. Seasonality of vegetation in eastern Australia’s forests can give rise to a pattern of when bats are most likely to be seeking food in human-populated areas. From 1994-2010, 14 Hendra virus outbreaks occurred, 12 of which were during the dry season in the southern hemisphere from May to October (also encompassing all of winter). During this time, natural vegetation growth is at its lowest, making it likely that bats will venture into urban regions. Some districts were experiencing droughts when spillovers occurred, and others were not, so temperature or precipitation extremes within a single season were not good predictors of spillover 7. However, the dry season observation is compelling enough to warrant increased surveillance for Hendra virus at that time.
Mating season, during which many bats come together and socialize, and when juveniles begin to lose antibodies passed from mothers could also increase the amount of virus the bats release in their saliva and excrement. This can lead to a waxing and waning pattern of outbreaks with seasons. Habitat loss can also change bat migration patterns and Increased urbanization also separates bat populations from each other, and in the coastal cities of eastern Australia, more daytime bat roosts have appeared in the past 2-3 decades, putting bats and humans closer to each other 8.
Nipah Virus Emergence
Malaysia and Singapore
The first known outbreak of Nipah virus (NiV) began in September 1998 in Malaysia and spread to Singapore. The virus was transmitted from flying foxes to domestic pigs, who then transmitted it to humans who worked in the livestock industry. To contain the outbreak, 1-1.5 million pigs were killed, ending the outbreak in May 1999. In that time, 265 people were infected in Malaysia, of whom 105 died. In Singaore, 11 people were infected, and 1 died, giving an overall case fatality rate of 38%. Many doctors initially guessed Japanese encephalitis to be the cause of the outbreak, which delayed identification of the novel virus and containment measures. Japanese encephalitis can cause permanent neurological damage and has a case fatality rate close to 30%, but vaccines are available 9.
Almost all NiV cases in this outbreak were directly associated with pigs, suggesting that Nipah virus in Malaysia is either unable or unlikely to spillover from bats directly to humans. The 11 cases in Singapore were all in slaughterhouse workers, many pig farmers in Malaysia fell ill, and culling of pigs (in addition to other human containment measures) ended the outbreak. In this outbreak, pigs were the amplifier host that allowed the virus to replicate to high levels before jumping to humans. A serologic (antibody) survey in Tioman Island, Malaysia (~2000 residents) showed that of 153 people, none had evidence of past NiV infection, despite living near Pteropus hypomelanus populations in which NiV has been found 10. The authors of this study concluded that Nipah virus in Malaysia can not directly spill into humans.
Bangladesh and India
Starting in 2001, a distinct strain of NiV has repeatedly spilled into humans in Bangladesh, causing outbreaks almost every year until 2015. Several districts have been affected, and in 2001, cases were recorded in the state of West Bengal in India, which borders Bangladesh. Separate outbreaks have been reported in Kerala in western India in 2018 and 2019, suggesting a wide range of NiV in Southeast Asian bats. Approximately 70% of infected individuals in Bangladesh and India died, which is significantly higher than the case fatality rate of the 1998-1999 outbreak. See here for an interactive plot of Nipah fatality rates, as well as case and death counts in all outbreaks.
NiV-M (Malaysia) and NiV-B (Bangladesh) share more than 90% nucleotide similarity, but have significantly different fatality rates and varying clinical presentation, making for an interesting conundrum 11. In addition, human-to-human transmission was evident in Bangladesh because close contacts of early cases fell sick as well, but not in Malaysia, where pig-to-human transmission dominated. In addition, pigs were a necessary amplifier host for NiV-M, but NiV-B was transmitted directly to humans via fruit products contaminated with bat fluids.
Philippines
In 2014, another NiV outbreak occurred in the Philippines, during which 9 out of 17 confirmed individuals with NiV infection died (case fatality rate of 52%). At least 10 dead horses were found, and several domestic dogs and cats that had eaten horse meat also died. Seven of the 17 infected humans had participated in butchering and eating horse meat, and 3 more had only eaten the meat. Five more cases, including 2 health care workers, had been in contact with other confirmed human cases, but not with any horses. These details mean that horses are an amplifier host and that human-to-human transmission occurred. Human and dog blood samples had neutralizing antibodies against Henipaviruses, but not against other common infectious agents. Since NiV and HeV are very similar, there were also some anti-HeV antibodies.
A serum sample from one patient was positive for NiV by real-time PCR, and a short stretch of one of the NiV proteins was sequenced and found to share 99% nucleotide similarity with NiV-M and 94%–96% similarity with NiV-B. However, since the sequence analysis was based on a single protein, it is not entirely certain to which strain the Philippine isolate is most similar. 12
Nipah Virus Strains
NiV infection by the two known strains can be distinguished somewhat by symptoms. NiV-M primarily caused neurological symptoms, such as dizziness, confusion, involuntary muscle jerks, and encephalitis. Pneumonia was reported in some patients in Singapore, but most cases in this outbreak did not have significant respiratory symptoms 13. It is believed that NiV can spread by droplet transmission, so few patients with NiV-M coughing may partially explain the lack of human-to-human transmission in the Malaysian outbreak. NiV-B has a shorter incubation period, and more patients experienced vomiting and difficulty breathing 14,15. In the Malaysian and Philippine outbreaks, pigs and horses were considered amplifier hosts, while in Bangladesh and India, people could contract NiV directly from date palm sap infected with virus from bats. There were no cases from human-to-human transmission in Malaysia and Singapore, but these were documented in the Philippines and Bangladesh.
The differing case fatality rates of NiV-M and NiV-B have been corroborated by studies in African green monkeys. In one study, all 4 monkeys infected with NiV-B died, while 2 of the 4 infected with NiV-M survived 16. Because of cost, research studies in primates are often very small. The lungs and spleens of the NiV-B-infected animals also showed more damage. When a neutralizing antibody was administered to the monkeys at different times after infection, the authors also found that there was a narrower window for NiV-B infection during which the antibody was protective.
Preparing for Future Henipavirus Spillovers
Hendra virus has not been documented to transmit from bats to humans, so most interventions focus on reducing contact between bats and domesticated animals like horses. This means monitoring bat roost sites and surveying them for present or past HeV infection so that horses can be kept away from them. Bats are difficult to study because they are small, elusive, and are typically found in hard to reach caves, but the 2018 NiV outbreak in India particularly highlights the need for ongoing and increased surveillance 17. Previous NiV outbreaks in India in the early 2000s occurred in the eastern part of the country near Bangladesh, presumably because NiV-carrying bats in Bangladesh are found across the border. However, the Kerala outbreak and subsequent discovery that bats in Kerala are sero-positive (have antibodies) for NiV demonstrate that NiV is likely to be found wherever Pteropus bats range, as shown below. It can very likely spill into humans in a place that has never seen an outbreak before.
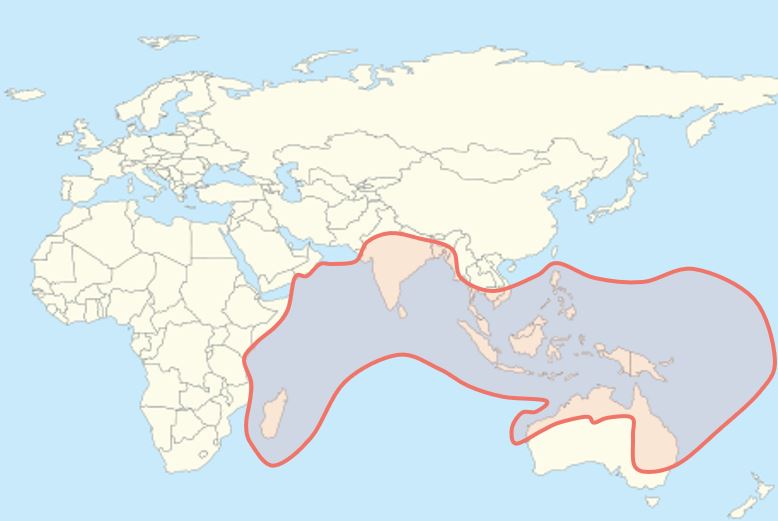 Flying foxes, which are part of the Megabat family, are found in Southeast Asia, Australia, Oceania, and parts of eastern Africa. Their diet consists primarily of fruit and other plant matter, but some eat insects as well. They can be distinguished by their dog-like faces and are the largest bats in the world (the largest species is the Giant Golden-Crowned flying fox from the Philippines).
Flying foxes, which are part of the Megabat family, are found in Southeast Asia, Australia, Oceania, and parts of eastern Africa. Their diet consists primarily of fruit and other plant matter, but some eat insects as well. They can be distinguished by their dog-like faces and are the largest bats in the world (the largest species is the Giant Golden-Crowned flying fox from the Philippines). Date palm sap is the putative fomite (an inanimate object that can transmit an infectious agent) that transmits NiV from bats to humans. It is a delicacy in Bangladesh that can fetch high prices to supplement people’s income. However, fruit bats feed on the same sap and roost in the same trees, and their urine, saliva, and other fluids can contaminate sap collection jars. Covers/skirts made of bamboo and other materials can prevent bat fluids from getting into jars 18, but this has proven expensive and difficult to implement on a large scale in Bangladesh 19.
Vaccines and Therapeutics
A Hendra virus vaccine for horses was developed in November 2012 by Zoetis under the name Equivac® HeV 20. It is a subunit vaccine composed of the surface G glycoprotein and was shown to elicit neutralizing antibodies that protected against infection and disease for 6 months 21. Equivac is licensed for use in horses, but not in humans, and the Australian Veterinary Association now recommends, but does not require, that all horses in Australia are vaccinated. Unfortunately, a study of 376 horse owners in Australia showed that 43% did not vaccinate their horses against Hendra virus 22. Mistrust of veterinarians and belief that the risk of Hendra virus infection is low were some of the most common predictors of non-vaccination. Horses require booster vaccines every 6-12 months to maintain immunity, but this has also led to conflicts with horse owners.
Current NiV research is performed only on two isolates -- NiV-M and NiV-B because so few people have contracted NiV, thus limiting research because samples were not collected, particularly in resource-poor areas. Furthermore, NiV infection progresses extremely rapidly in patients, making it difficult to study its pathogenesis. Animal models of disease have been developed (mainly ferrets, hamsters, and non-human primates), but due to the high lethality of NiV, research must be performed under biosafety level 4 (BSL-4) conditions and is difficult.
Antibodies isolated against NiV have increased the survival of animals treated with them 23,24. This suggests that passive transfer of serum can have beneficial effects as a treatment in the absence of a vaccine. Ribavirin, a molecule that induces errors when RNA viruses copy themselves, was tested during the 1998-1999 Malaysia outbreak. A smaller proportion of deaths was recorded in the group that received the antiviral (note that the patients in the control group either refused ribavirin or were treated before the study) 25. Four African green monkeys treated with remdesivir, another antiviral that interferes with viral replication, all survived NiV infection, while the four untreated ones did not 26.
Based on our knowledge that at least 4 NiV proteins impair the innate immune response, adjuvants, which are compounds that promote an immune response, could reduce disease severity. Administering double stranded RNA, which is a foreign compound to cells that stimulates antiviral and inflammatory activity, to hamsters starting when they were infected with NiV prevented severe disease in 5 out of 6 hamsters 27.
Some NiV vaccine candidates in preclinical development have performed well in animals. Most are subunit vaccines composed of a NiV protein, typically the attachment glycoprotein 27. Others place the surface proteins of NiV onto another virus to present them to the immune system 28. These vaccines protected animals from lethal NiV infection and could be administered to humans and domestic animals once appropriate trials have been conducted.
Conclusion
Although we are still in the thick of the COVID-19 pandemic, it is important to remember that numerous other zoonotic viruses are evolving all the time. Preparedness, surveillance, knowledge of microbial threats, protecting wildlife, and reducing meat consumption and the wildlife trade will reduce the risk of a future pandemic crippling the entire world. Henipaviruses have already been identified as a significant threat, so we can not wait for Nipah and Hendra viruses to evolve to become efficient at human-to-human transmission before we develop effective response plans, treatments, and vaccines.
The international organization EcoHealth Alliance works to predict and prepare for future pandemics by studying interactions between humans and the environment and mitigating risks, including reversing deforestation and promoting wildlife conservation and biodiversity. With such approaches, we can improve pandemic preparedness and build a more sustainable future.
Leave a Comment