The Age of Zoonoses
Pathogens are zoonotic if they are transmitted from animals to humans. Zoonotic viruses have recently been brought to the world's attention by SARS-CoV-2, but their pandemic potential has long been recognized. In 2012, seven years before the start of the COVID-19 pandemic, David Quammen published Spillover, a book about several prominent zoonotic pathogens. He spent years interviewing and accompanying field epidemiologists, virologists, veterinarians, doctors, and others about emerging zoonoses, as well as intriguing nuances of familiar threats like malaria. It is a phenomenal book, and I highly recommend it.
Zoonotic Spillovers
Many pathogens that infect humans originated in animals and crossed to humans in a spillover event. These bacteria and viruses are the most difficult to eradicate because even if all humans are immune at a given time, the infectious agents will continue to survive in another animal -- called a reservoir host. Viruses such as smallpox and polio are not zoonotic, so they can more easily be eradicated because they are not found in any species.
The most common animals from which humans acquire pathogens are bats, rodents, birds, and other primates because of extensive direct or indirect contact with them. Bats are the reservoir hosts of coronaviruses, Rabies, Nipah, Hendra, Marburg, and probably Ebola viruses 1. The human immunodeficiency virus (HIV) originated in African primates, hanta- and lassaviruses are found in rodents, and West Nile virus and influenza came from birds. A reservoir host harbors a pathogen, but does not suffer from the disease. It is difficult to prove that an animal is a reservoir host because a pathogen must be isolated from an animal without symptoms and grown in the laboratory. This demonstrates that a replicating pathogen is found in the animal, but is not causing disease in that animal.
A bacterium or virus that infects one animal may not necessarily be able to infect others. Domesticated animals like horses, pigs, cows, and chickens are intermediate hosts that often help pathogens transmit from wild animals to humans through an intermediate animal.
The Perfect Storm
A large amount of very interesting ecological and microbiological detective work has been undertaken in the past several decades to determine the reservoir hosts of many new pathogens. As humans increasingly alter natural ecosystems and come into contact with animals, the risk of bacteria and especially viruses finding a new home in humans is growing. Favorable ecological circumstances allow infectious agents to get into a new host, and evolution allows them to become established there.
There have been many spillover events throughout human history. For most of human history, people frequently succumbed to microbial infections without knowing what caused them. Viruses are so tiny that they slip through even the smallest laboratory filters, and the first human virus (yellow fever) was isolated only in 1901 2. Before modern times, the human population was much less dense, and we lacked the means to travel quickly and over great distances, so an outbreak in one group may not have turned into a pandemic.
Many emerging viruses are zoonotic because the human population is growing exponentially, causing more ecosystem disturbance and animal habitat infiltration. In order to propagate, viruses require many susceptible hosts, so although humans have always been in contact with animals, viruses have never had such an easy time spreading between individuals. Domesticated animals today live in closer quarters than their wild counterparts, further facilitating spread between animals and to humans. Globalization and the rapid movement of humans allow an emerging virus to be transported all over the world in a matter of hours, as we have seen with SARS-CoV-2.
Human Immunodeficiency Virus (HIV), the Great Modern Plague
Most people are familiar with HIV and the disease it causes, acquired immunodeficiency syndrome (AIDS). HIV attacks and kills certain immune cells that are responsible for fighting off infectious. Once these cells die, a person becomes hypersusceptible to infections, even those that are relatively harmless to the general population. Left untreated, HIV infection progresses to AIDS in 8-10 years on average, but improved antivirals like cocktails of drugs called highly active antiretroviral therapy (HAART) reduce this risk significantly 3.
HIV could have spilled into humans multiple times, but these were “dead-end” infections that weren’t spread widely to other humans. Only one strain caused a pandemic because conditions were right for widespread transmission, like urban population growth in Africa. This strain is called HIV-1 group M (M for “main”), and the most closely related virus is a type of simian (monkey) immunodeficiency virus (SIV) that was discovered in chimpanzees in Cameroon 4. There are many SIV variants, and in the scientific literature, they are distinguished by a 3 letter subscript denoting their animal of origin.
The other main strain is called HIV-2, and it spilled in West Africa from another African primate called the sooty mangabey, which carries SIVsmm. HIV-2 is less infectious than HIV-1, causes disease slower and with a lower mortality rate, and is largely confined to West Africa 5. Sooty mangabeys and African green monkeys infected with SIV species did not display any symptoms of disease, and for a long time, it was believed that SIVs in other non-human primates were similarly nonpathogenic. However, in 2009, a team of researchers showed that chimpanzees infected with SIVcpz suffer greater mortality rates than uninfected individuals 6.
It is likely that SIVcpz is to chimpanzees as HIV is to humans. That is, chimpanzees acquired SIVcpz from another African primate and suffer as humans do from immunodeficiency. Chimpanzees are omnivorous and sometimes prey upon smaller monkeys, which could have provided the opportunity for them to be in contact with infected blood from a SIV reservoir host. This is the same “cut hunter” hypothesis of how a human acquired SIVcpz from a chimpanzee while hunting and butchering bush meat. Blood from the chimpanzee could have dripped into a wound on the hunter, transmitting the virus. Due to mutations acquired over the course of replication and evolution in humans, SIVcpz and HIV diverged, just as the many groups and subtypes of HIV-1 separated from each other.
Coping with Influenza
In a previous post, I discussed some phenomena for which influenza virus is a textbook example, such as original antigenic sin, immunodominance, and viral genome reassortment. Influenza is a lethal zoonotic virus that has plagued humans for millennia. Unlike HIV, it is much more easily spread, but we have reasonably effective vaccines against seasonal influenza. Influenza originated in aquatic birds and most likely spilled into domestic poultry when humans began domesticating animals between 8000 and 2500 B.C. This also means that avian influenza strains must have some adaptations that allow them to persist and be transmitted in water, which is a frightening thought.
Situations that bring large numbers of different species into close proximity provide the perfect conditions for a zoonotic spillover. Wet markets in Southeast Asia and the livestock industry around the world put animals into cramped conditions, harming their overall health and making them susceptible to infections and disease. In these conditions, viruses can spread unchecked between species, which can be good amplifying or mixing hosts. Finally, slaughtering animals en masse furthers disease spread because pathogens are transmitted through infected fluids.
Pigs are good mixing hosts because they can be infected simultaneously with human and avian influenza, allowing genome reassortment and the emergence of novel influenza strains 7. Current animal surveillance shows that influenza A, the most serious type in humans, can be isolated from many warm-blooded animals. Recently, an analysis of 7 years of data demonstrated that the prevalence of a reassortant swine influenza in domestic pigs in southern China is increasing 8,9. In fact, swine flu is not required to be reported to the United States government 10, despite knowledge that swine influenza strains circulate in global pig populations and that pigs are a significant source of novel influenza viruses in humans.
Why Do Bats Harbor So Many Viruses?
Bats are the reservoir hosts for a surprising number of RNA viruses, including Nipah, Hendra, Marburg, corona-, and probably Ebola viruses. Bats have extensive geographical ranges, and because they fly, they can travel great distances relatively quickly. Since they are ubiquitous in many climates, they can easily come in contact with humans or livestock. Bats live in communal societies at extremely high densities, facilitating the spread of infectious diseases. They frequently move through different permanent roost sites, suggesting that they travel and come in contact with bats of other populations. All of these features make bats good at spreading diseases among themselves and to humans and other animals.
The World of Bats
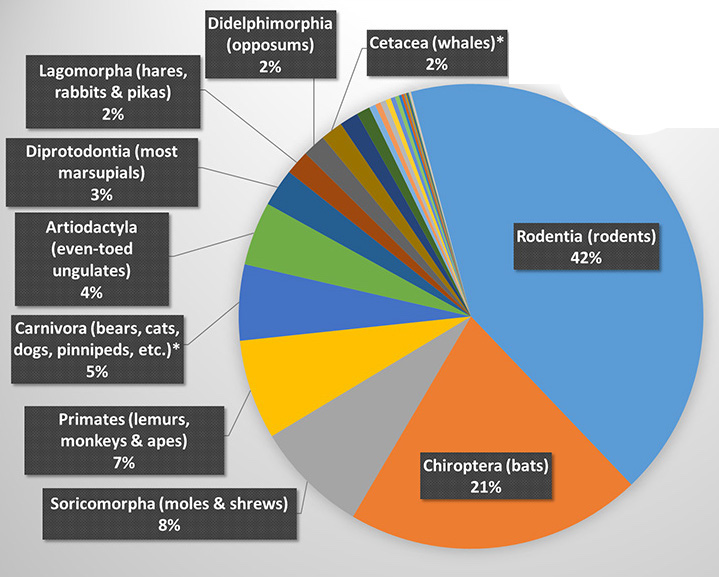
Bats are mammals that make up the order Chiroptera (chiro = “hand” and ptera = "wing" in Greek). Chiroptera is the second most diverse order of mammals, following Rodentia (rodents), and together, they account for more than half of all mammal species diversity, as shown in the figure on the right. Source: University of Hawaii at Manoa
Bats are the only mammals capable of flight, joining birds and pterosaurs -- extinct reptiles related to dinosaurs -- in the group of flying vertebrates. Unlike flying birds, bats do not have hollow bones, but their bones are nevertheless slender and delicate to reduce their weight. The smallest bat species known is the Kitti's hog-nosed bat, weighing in at only 2 grams, and the largest bats are flying foxes, which weigh approximately 1.5 kilograms and have wingspans up to 1.7 meters long. Phylogenetically, bats are separated into the suborders Microchiroptera and Megachiroptera based on size and evolutionary relationships. Megachiropterans are Old World fruit bats, and contrary to popular belief, they are incapable of echolocation.
Bats are native to every continent except Antarctica. Southeast Asia, Central and South America, and Africa have the highest bat diversities by species because bats (and biodiversity as a rule) thrive in tropical, subtropical, and temperate climates. In cold climates in North America and Europe, some bats hibernate in large roosts to conserve body heat, while others migrate to lower latitudes for the winters 11. Popularly known as “vampire bats,” only 3 of the more than 1,400 known species of bats feed on animal blood. Moreover, the bats most often responsible for lethal virus spillovers are fruit bats and insect-eating horseshoe bats. So blood-sucking is hardly part of the equation when it comes to bat-to-human spillovers.
Bat Immune Systems
Lethal RNA viruses generally trigger overactive inflammation leading to death in humans. The virus itself doesn’t kill you, but the induced hyperinflammation causes irreversible tissue damage, which, if it occurs in vital organs, can cause organ failure and death. Thus, the leading hypothesis for how bats tolerate these viruses is adaptations in their immune system.
There are many biochemical pathways in the innate immune system that are responsible for producing inflammatory molecules at the onset of an infection. Sensor proteins called pattern recognition receptors recognize molecules that are typically found in microbes or are produced by cells when they are damaged. These receptors function as sentinels to alert the immune system that something is wrong and bring immune cells, clotting factors, and proteins (all found in blood) to a wound or infection site. Bats’ ability to tolerate so many viruses without suffering the same symptoms as humans may be due to a dampened inflammatory response. Bats tend to have high viral loads in their tissues, making them very good at shedding virus in their urine, feces, and saliva, but they do not show clinical signs of disease 12.
A very interesting paper from the Wuhan Institute of Virology showed that a single amino acid change in a protein called STimulator of Interferon Genes (STING) may reduce virus-induced inflammation in bats. STING is involved in a cellular pathway that senses the presence of DNA in the cytoplasm of a cell. In eukaryotic cells, DNA is found only in the nucleus and the mitochondria, and so cytoplasmic DNA is a sign of cellular damage, infection by a microbe, or cancer. STING activation eventually leads to the production of type I interferons, which have antiviral and inflammatory activity. But the STING versions most commonly found in many bat species produced less interferon in bat and human cells cultured in a lab. When the bat STING was modified at one position to resemble human STING, more interferon production was observed. 13
TNF-α is a proinflammatory cytokine produced by many innate immune cells and causes fever and inflammation. A 2017 study found that although Eptesicus fuscus (big brown bat) and human cell lines exhibited similar antiviral activity, the bat cells produced much less TNF-α than the human cells when stimulated with a double stranded RNA analog (double-stranded RNA is also an anomalous molecule in cells). This was due to a protein that represses gene expression present in front of the TNF-α gene, so some bats produce less TNF-α. 14
A protein complex called an inflammasome is a cluster of many proteins involved in stimulating the production of proinflammatory cytokines. The NLRP3 inflammasome is an important sensor that recognizes both cellular stresses, such as extracellular adenosine triphosphate (ATP), mitochondrial damage, and oxidized DNA, an bacterial or viral RNA in the cytoplasm. Overactivation of NLRP3 has been linked to hyperinflammation and immunopathology in response to infections by influenza, rabies, and other viruses. Immune cells from Pteropus alecto (black flying fox) showed less NLRP3 activation than human and mouse cells when infected with MERS coronavirus and influenza virus, but there were no significant differences in viral loads across the species. 15
There are many other differences between the innate immune receptors and genes of humans and bats since they have been evolving separately for vastly different lifestyles for millions of years. As mentioned before, there are more than 1,400 known species of bats, and adaptations in one species for coexistence with viruses may not necessarily be shared by other species. Many adaptations likely also work in concert, complicating the matter further. However, it is clear that bats can be infected with many types of viruses without mounting self-damaging immune responses.
Hypotheses on Bat Evolution
How did bat immune systems get to be like this? One hypothesis is that the dampened inflammatory response co-evolved with bats’ high metabolic rate. The hearts and lungs of bats are larger than those of terrestrial mammals of similar size, and their blood delivers more oxygen per heart beat. Because of their small size, their metabolic rate per unit mass during flight is very high, and their resting metabolic rate (non-flying) is 3-5 times greater than that of other mammals 16. Flying is extremely taxing metabolically, and similar trends hold true for birds.
Extremely high metabolic load ramps up oxidative phosphorylation, the process by which adenosine triphosphate (ATP) is produced in the presence of oxygen to provide energy to cells. This in turn leads to the production of oxygen radicals, which are toxic to cells because they induce DNA damage. Consequently, some scientists hypothesize that to cope with very high levels of oxidation-induced DNA damage, bats evolved dampened inflammatory responses. Elevated metabolic rates are associated with shorter lifespans due to inflammation-induced cellular damage, but bats have surprisingly long lifespans that could be a reflection of dampened inflammation 17,18. Bats live to averages of 10-20 years and some up to 40 years, while most similarly-sized rodents live fewer than 10 years and most fewer than five.
Flying raises the metabolic rates of bats approximately 15 times that of their resting rate 19, whereas for birds and running rodents, the corresponding figures are 2-fold and 7-fold, respectively 20. Strains of laboratory mice bred for higher metabolic rates produced more antigen-specific IgMs (the first antibody produced in an immune response) and had more white blood cells than strains bred for lower metabolic rates 21. A higher average metabolic rate in bats due to the time spent flying could put them in a chronic state of immune elevation, just as fever in humans enhances immune function, including mobility and cytokine secretion. In addition, the large increase in metabolic rate between roosting and flying could allow immune cells to respond faster. During flight, bat internal temperatures vary from 37-42oC, depending on the species, and 38-41oC is the typical range for mammalian fever 20. All of this could make bats more tolerant of viruses, as well as select for viruses capable of surviving high host temperatures and large temperature fluctuations.
Bats aren’t immune to all pathogens. The infamous white-nose syndrome, which is caused by the fungus Pseudogymnoascus destructans, is devastating North American bat populations. The fungus attacks bats while they are hibernating 22. During this time, the slower metabolic rate of bats may allow the fungus to get a foothold and spread unchecked 23. The fungus causes the bats to wake up prematurely, covered in white fuzz and with damaged wings that contribute to the disruption of bats' water and electrolyte balance. The sudden increase in body temperature when bats wake up causes immune cell proliferation and inflammation-mediated pathology 23. Bats in Europe and Asia carry the fungus but do not get nearly as sick as their North American counterparts, suggesting that they may have co-evolved with it 24. Since bats do not perform trans-Atlantic migrations, it is likely that human activity transported the fungus, which can survive on surfaces like cave walls before infecting bats.
What About Birds and Rodents?
If the high metabolic rate due to flying could have facilitated bats’ coexistence, it is natural to wonder if this same phenomenon is at play with birds. As mentioned before, influenza A originated in wild aquatic birds, but birds are also the reservoirs of numerous bacterial and fungal pathogens. These include a species of Mycobacterium (the causative agent of tuberculosis), Chlamydia bacteria that cause parrot fever (psittacosis), Salmonella, Campylobacter, and Cryptococcus fungus 25. These are transmitted to humans typically via contact with infected bird droppings, soil, or poultry.
Another virus originating in birds is West Nile virus, which is transmitted by biting mosquitoes. It is endemic in birds in Europe, Africa, and Asia and is non-pathogenic in them, but causes significant mortality in birds in the Americas 26. It was first identified in a Ugandan woman in 1937 27, but the first reported human case in the Western Hemisphere was in 1999 in New York City 28, where it infected both wild and captive birds in addition to humans. A group of coronaviruses called gamma-coronaviruses primarily infects birds. Thus far, only alpha- and beta-coronaviruses from bats have been able to infect humans.
Rodents are another group of vertebrates that harbor many pathogens, including hantaviruses, lassa virus, and lymphocytic choriomeningitis virus, and numerous bacteria, such as Salmonella, Campylobacter, Yersinia pestis (plagues), Borrelia spirochetes (Lyme Disease), and the infectious agents of rat bite fever and tularemia 29. Close contact with the animals or their urine, saliva, and feces, bites and scratches, and contaminated food and water are the most common routes of transmission of rodent-borne diseases.
There are many more pathogens not listed here that infect animals. Most of these are not zoonotic because they can’t infect humans, but this could change. Bats harbor more viruses per species than rodents (but there are more rodent species, so collectively more rodent viruses) 30, and since viruses do not have any reliable treatments like antibiotics for bacterial infections, bats are the likely origin of future pandemic viruses. The immune systems of birds have not been well studied, and although laboratory mice are well characterized, wild rodent species are not.
Conclusion
When a pathogen emerges into a new host population, two things can happen. 1) The pathogen kills off the host, leaving little evidence of the event, or 2) the host population manages to survive (it could still sustain significant losses), and the pathogen becomes endemic in that population. It is not true that all pathogens mutate to be more virulent or less virulent. Different pathogen characteristics could be favorable depending on the environmental conditions and behavior of hosts. HIV is very lethal, but difficult to transmit, while SARS-CoV-2 is very transmissible, but much less deadly. Both have become pandemics and infiltrated every corner of the human population.
We have entered the age of zoonoses. We disrupt natural habitats, breed massive numbers of livestock for food (which is detrimental to both the environment and public health), engage in risky behaviors such as visiting bat caves and eating exotic wildlife, and can travel around the world in less than a day, giving pathogens ample opportunity to infect many hosts before they are detected. It is inevitable that more pandemics like COVID-19 will occur in the future, and when they do, we must be prepared with swift behavioral modifications and policies for controlling spread, a stockpile of antivirals and antibiotics with broad activity, and effective surveillance methods to identify the sources of outbreaks. If not, then we have proven that we do not learn from history.
Leave a Comment